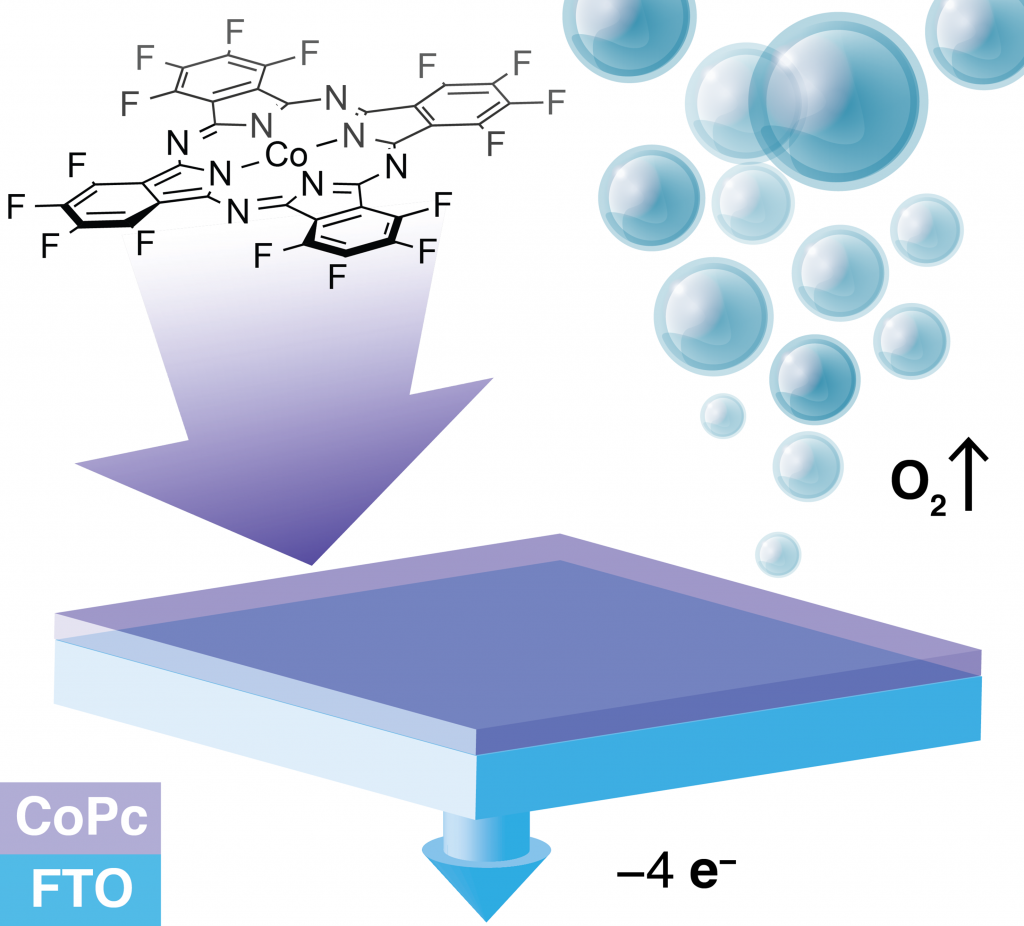
The development of effective and stable catalysts for electrochemical conversion of renewable small-molecule feedstocks, such as water, CO, and CO2, is one of the main challenges in the establishment of a practical solar fuel scheme. Heterogeneous metal-oxide catalysts have been the most popular in this context due to their stability and accessibility. The application of molecular catalysts in this context has been restricted by their synthetic complexity and limited stability. Enzymes rely on the localization of hydrophobic and hydrophilic domains to achieve optimal conformations. We aim to exploit the phenomenon of hydrophobicity to mediate the interactions between molecular electrocatalysts and supporting inorganic surfaces. For suitably designed molecules, their hydrophobicity can ensure that they will never desorb into an aqueous phase, and will be conformationally locked. These characteristics can make the ultra-hydrophobic molecules extremely stable even under harsh oxidative conditions on the anodes.